Each chemical element has an atomic number that shows the number of its protons (positively charged particles) and electrons (negatively charged particles) when the element is in a stable (non-ionized) state, as well as the mass number or neutron number. Depending on their number, some elements have variations called isotopes. The number of neutrons shows the evolution of elements, electrons, and protons, and the course of electricity.
In this article you will learn:
- What shapes atoms can have.
- How protons and neutrons are arranged in the nucleus of an atom.
- The law of the octaves of chemical elements.
Since antiquity, mankind has tried to connect the structure of the universe and its constituent parts, i.e. the elements, with geometry. Ancient alchemists as well as Pythagoras believed that the 5 Platonic solids contained aspects of all the elements. They defined the basic elements that make up our world, that is, what we now call chemical elements.
Among the alchemical elements are:
- Fire – Helium – Tetrahedron
- Earth – Carbon – Hexahedron
- Air – Oxygen – Octahedron
- Water – Hydrogen – Icosahedron
- Ether, prana – Nitrogen – Dodecahedron
In 1895, in England, a group of clairvoyants made a study of the structure of elements and chemical compounds using the method of mental clairvoyance.
The results of their work were published in the work entitled “Occult Chemistry. Investigations by clairvoyant magnification into the structure of the atoms of the periodic table and some compounds.” As a result of their research, they discovered that the elements have certain geometric shapes. With a few exceptions, each belongs to one of the seven forms they have named:
- Spire
- A dumbbell
- Tetrahedral – here one should e.g. oxygen
- Cubic – nitrogen belongs to this group
- Octagonal – this group includes, among others carbon
- Barrow – is a group of inter-periodic elements, metals
- Starry – these are noble gas atoms. They are in the form of six-pointed stars, but in the atoms of the same element, all the arms of the star have the same internal structure, which is the 6 Vesica Piscis of the Flower of Life.
It also turned out that each atom has a spherical shell. All the chemical elements except hydrogen, oxygen, and nitrogen are built like Platonic solids: tetrahedron, cube, octahedron, dodecahedron, and icosahedron. During their research, they also carried out measurements of the atomic mass, which they then verified with the research of scientists, and it turned out that they obtained the same results.
Platonic solids.

Platonic solids.
According to modern science, chemical elements are collections of atoms.
Atoms, on the other hand, are spectra or shells or energy thresholds on which electrons are arranged. There can be 7 of these shells, or collections of orbitals, just like energy changes in the spectrum of light. This number results from the geometry of space and the number of spheres that come into interaction with each other.
Just as each frequency level of light produces a new quality – color, each new electron shell produces a new group of substances on the periodic table. All these shells are interdependent, and each atom strives for completeness and fullness, i.e. obtaining 8 electrons on the last of them. When this shell is full, a new further shell forms. The number of electrons on the last shell called valence determines which family (group) of the element belongs to:
Seven electron shells and the maximum number of electrons on them:
- K – 2 electrons
- L – 8 electrons
- M – 18 electrons
- N – 32 electrons
- O – 50 electrons
- P – 72 electrons
- Q – 98 electrons
Element families:
- Alkali metals – 1 valence electron
- Alkaline earth metals and transition metals – 2 valence electrons
- Boron group or earth metals – 3 valence electrons
- Carbon group or Tetrel – 4 valence electrons
- The nitrogen group of pnictogens – 5 valence electrons
- Chalcogen oxygen group – 6 valence electrons
- Halogens – 7 valence electrons
- Noble gases (neutral) – 8 valence electrons
The English chemist John Newlands, while arranging the elements according to the increasing atomic masses, noticed that every eighth element has similar properties. He called this discovery “the law of the octaves.” It confirms the layered system of fractals in which each successive layer somehow reflects the basic one and contributes to Mendeleev’s law of periodicity.
The electron shells are probability clouds.
The protons in the nuclei of atoms, on the other hand, are also organized in shells in the nucleus. Each of them is a vertex of Platonic solids. What the American nuclear physicist and chemist Dr. Robert Moon proved in his research. In his opinion, more than one geometric form can be nested in the nucleus at the same time – each in the other.
The first form is a string made of 2 helium protons that form a sphere. After the decay of helium, a point is formed, i.e. one hydrogen proton being the center of the next sphere, and then another point isotope hydrogen-deuterium – the center of the next sphere, and another point – the hydrogen isotope tritium the center of the third sphere. This leads to the formation of another string of helium, and then lithium with 3 protons.
This creates a tetrahedron with four beryllium protons at its vertices. Beryl creates a prism – a spatial triangle, a symbol of God. Another proton forms the boron, the next figure is an octahedron made of the six protons of the carbon nucleus.
Moon claimed that the first stable structure was a cube, and its vertices determined the distribution of 8 protons in the oxygen nucleus.
Another 6 protons formed another shell, which formed the body of the octahedron, which corresponds to the distribution of 14 protons in the silicon nucleus. The next 12 protons form the icosahedron shape corresponding to the iron nucleus and its 26 protons. The next 20 protons make the dodecahedron or 46 protons of the palladium nucleus.
After the four shells are completed, the successive elements begin to form a twin structure on top of one of the twelve faces of the dodecahedron. Both structures share five vertices of this wall and an icosahedral vertex in its center. The next block will be a dodecahedron reflecting the distribution of 56 protons in the barium nucleus. The next 8 protons complete the inner hexahedron giving rise to a gadolinium nucleus with 64 protons. Another element with 70 protons is the ytterbium which forms the octahedron shape.
The next 11 protons are the vertices of the icosahedron in the 81st proton nucleus of the thallium. The icosahedron is then formed around the cube-octahedron structure, ending its vertices in the lead (82). Lead is the stable endpoint of a series of radioactive decay. Another 5 protons close all shells with the element radon with 86 protons.
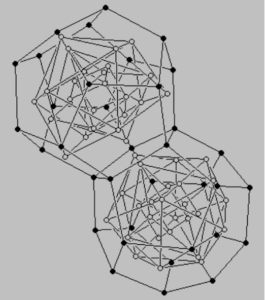
When the entire structure is filled, the dodecahedrons separate as if they were hanging on a hinge.
In this way, 4 spaces are freed, which are to be occupied by an additional 4 protons. Overloading the structure with successive protons is unstable, therefore the non-naturally occurring elements Frans (87 protons) and actinium (89 protons) cause the “hinge” to break. This causes the structures to stay on one point.
And another proton makes a protactinium nucleus with 91 protons. Then, at the junction point, another proton from the uranium nucleus is formed with 92 protons. The resulting structure is ready to be split open, and the extra neutrons will cause it to break. Uranus also has 92 electrons and 146 neutrons.
Neutrons occupy the midpoints of the edges of nested Platonic solids and the center of icosahedral faces that are not occupied by protons.
Since the edges of the solids are the diameters of the spheres that make up the Flower of Life, the neutrons are at the center of these circles. Moon’s associate Larry Hetch worked on the position of the neutrons. In his opinion, this concept is confirmed by the uranium-238 nucleus. In the uranium nucleus, the two dodecahedron structures interpenetrate each other, and the number of edges and wall centers left to be occupied by the neutrons is 73 neutron positions on each twin structure. Exactly the number of Uranium’s 146 neutrons.
Protons stay in place inside the nucleus by geometric waves, specifically the contact point of electric, magnetic, and sound waves.
These waves are shaped like Platonic solids. As in Cymatics, geometric waves hold the sand particles in place along the geometric lines of force of the flow. The same thing happens in the atom. The waves hold electrons and protons along the edges and corners of the geometry. We see geometry because the particles come together along the lines of geometry. This is the key!
We consider the protons inside the nucleus to be particles. Meanwhile, they are the vertices of wave geometry. When one geometric structure has finished building one geometry, and more energy is supplied to it, another geometric structure will begin to form around the first. For this reason, atoms can appear to be “waves” and “particles” at the same time. They may appear to be both because they are made of vibrating, fluid-like energy.
When you count the number of vertices for each of the Platonic solids:
- 8 points on the cube
- 6 on the octahedron
- 12 on an icosahedron
- 20 per dodecahedron = 46 total
This 46 is equal to the first half of the periodic table. In nature, however, there are a total of 92 elements (92 = 46 x 2).
Dr. Robert Moon was inspired by his research of Johannes Kepler’s concept of the solar system, described in the work “Mysterium Cosmographicum”.
Kepler was a German mathematician, astronomer, and astrologer. He discovered that if a regular octahedron is described in the sphere defined by the orbit of Mercury, it turns out that it is inscribed with the sphere of Venus. If, on the other hand, an icosahedron is described in the Venus sphere, it will be inscribed in the sphere of the Earth. The regular dodecahedron described on this sphere is inscribed in the sphere of Mars, and the regular tetrahedron described on the sphere of Mars is inscribed with the sphere of Jupiter. In turn, the cube described on the sphere of Jupiter is inscribed in the sphere of Saturn.
Thus, numerical harmony in the solar system was discovered. This harmony also translates into the musical harmony of the so-called planets – the harmony of spheres, i.e. the concept of Pythagoras, who believed that each planet in its orbit travels makes pleasant sounds, which we cannot hear, however. As you already know, he was right, and these sounds are made by the magnetic field of each planet.
Moon’s model is also inspired by von Klitzing’s discovery of the quantum Hall effect, leading Moon to believe that space itself is quantized and that nucleons are in discrete places, i.e. at the vertices of a set of nested platonic solids.
Source:
Laurence Hecht „The Geometric Basis for the Periodicity of the Elements”, 1988, 21st Century, www.21sci-tech.com
Jordi Solà-Soler „Sacred Solids in the Atomic Nucleus”, 2013, www.sacred-geometry.es
Renee Hoadley „Article 131: Atomic Chemistry – Part 2 – Periodic Table of Elements & Dr. Robert Moon”, www.cosmic-core.org